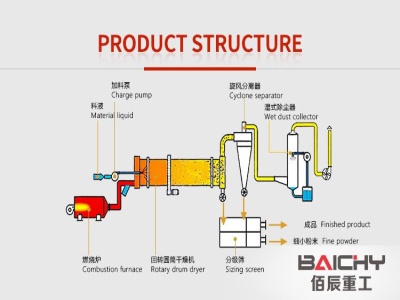
molar mass of iron iii oxide
The molar mass of iron(III) oxide is g/mol. (Round this to 160. g/mol, for 3 significant digits.) Divide this into the given mass to get the number of moles: (246 g) / (160. g/mol) = mol.